Article Contents ::
- 1 Details About Generic Salt :: Measles
- 2 Main Medicine Class:: Vaccine, live virus
- 3 (MEE-zuhls, mumps and roo-BELL-uh vaccine, live) M-M-R-II, Mo Ru-Viraten Berna Class: Vaccine, live virus
- 4 Drugs Class ::
- 5 Disclaimer ::
- 6 The Information available on this site is for only Informational Purpose , before any use of this information please consult your Doctor .Price of the drugs indicated above may not match to real price due to many possible reasons may , including local taxes etc.. These are only approximate indicative prices of the drug.
Details About Generic Salt :: Measles
Main Medicine Class:: Vaccine, live virus
(MEE-zuhls, mumps and roo-BELL-uh vaccine, live)
M-M-R-II, Mo Ru-Viraten Berna
Class: Vaccine, live virus
Drugs Class ::
 Action Induces protective antibodies against measles, mumps and rubella viruses.
Action Induces protective antibodies against measles, mumps and rubella viruses.
Indications for Drugs ::
 Indications Vaccination of individuals known to be susceptible to measles, mumps or rubella; prevention of occurrence of congenital rubella syndrome (CRS) mong offspring of women who contract rubella during pregnancy. Preferred immunizing agent for most children and many adults.
Indications Vaccination of individuals known to be susceptible to measles, mumps or rubella; prevention of occurrence of congenital rubella syndrome (CRS) mong offspring of women who contract rubella during pregnancy. Preferred immunizing agent for most children and many adults.
Drug Dose ::
 Route/Dosage
Route/Dosage
ADULTS & CHILDREN: SC 0.5 ml. Optimal schedule: Give first dose at 12–15 mo; revaccinate routinely at 5–6 yr or 11–12 yr.
Contraindication ::
 Contraindications Pregnancy; moderate to severe hypersensitivity reaction to eggs; immunosuppressive therapy; blood dyscrasia, leukemia, lymphoma of any type or other malignant neoplasms affecting the bone marrow or lymphatic systems; primary or acquired immunodeficiency; active untreated tuberculosis; family history of congenital or hereditary immunodeficiency, until immune competence of potential vaccine recipient is demonstrated.
Contraindications Pregnancy; moderate to severe hypersensitivity reaction to eggs; immunosuppressive therapy; blood dyscrasia, leukemia, lymphoma of any type or other malignant neoplasms affecting the bone marrow or lymphatic systems; primary or acquired immunodeficiency; active untreated tuberculosis; family history of congenital or hereditary immunodeficiency, until immune competence of potential vaccine recipient is demonstrated.
Exception: Vaccinate asymptomatic children with HIV infection.
Drug Precautions ::
 Precautions
Precautions
Pregnancy: Category C (contraindicated). Lactation: Excreted in breast milk (vaccine-strain rubella). Acute febrile illness: Defer immunization during course of any acute febrile illness.
|
PATIENT CARE CONSIDERATIONS |
|
Drug Side Effects ::
 Adverse Reactions
Adverse Reactions
CNS: Fever; headache; encephalitis; dizziness; polyneuritis; arthralgia; arthritis rarely chronic); convulsions or seizures. DERM: Urticaria; rash; erythema multiforme. EENT: Sore throat; optic neuritis. GI: Nausea; vomiting; diarrhea. HEMA: Thrombocytopenia; purpura. OTHER: Local pain, induration, erythema or allergic reaction at injection site; mild regional lymphadenopathy; malaise.
Drug Mode of Action ::
 Action Induces protective antibodies against measles, mumps and rubella viruses.
Action Induces protective antibodies against measles, mumps and rubella viruses.
Drug Interactions ::
 Interactions
Interactions
Human antibody products: To avoid inactivating vaccine, give MMR 2–4 wk before or 3–11 mo after AGIV, depending on dose. Susceptible postpartum women who received blood products or Rho(D) immune globulin may receive rubella vaccine prior to discharge, provided that rubella titer is measured 6–8 wk after vaccination to ensure seroconversion. Immunosuppressants, interferon, meningococcal vaccine: May inhibit response to MMR vaccine.
Drug Assesment ::
 Assessment/Interventions
Assessment/Interventions
- Obtain patient history, including drug history and any known allergies. Note untreated tuberculosis, history of immunocompromised disease, immunosuppressive therapy, or hypersensitivity to eggs, egg products or neomycin.
- Ensure that pregnancy test has been performed on sexually active women.
- Observe for local redness and warmth at injection site.
- Monitor for fever, dizziness, arthritis or rash. Notify physician if these symptoms occur.
- Record immunization in patient’s record.
Drug Storage/Management ::
 Administration/Storage
Administration/Storage
- If patient is febrile, delay administration if possible.
- Reconstitute using supplied diluent.
- With 25-gauge
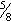 -inch needle, inject total volume of reconstituted vaccine SC, preferably into outer aspect of upper arm.
-inch needle, inject total volume of reconstituted vaccine SC, preferably into outer aspect of upper arm. - Refrigerate before and after reconstitution and protect from light.
- Discard unused reconstituted vaccine if not used within 8 hr.
Drug Notes ::
 Patient/Family Education
Patient/Family Education
- Advise patient that tenderness, redness, swelling and warmth at site of injection may occur.
- Applying warm compress to site will decrease these symptoms.
- Instruct patient to notify physician if local symptoms persist.
- Caution sexually active women to avoid pregnancy for 3 mo after vaccination.

